Continence Management Products
Continence Care
Continence Care
For information only, products are not available in Singapore.
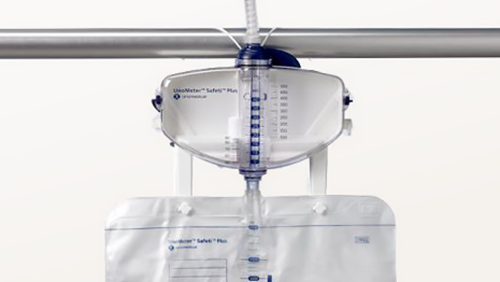
In an in-vitro study comparing products, only with UnoMeter™ Safeti™ Plus Urine Meter were bacteria not found at the bag to meter connection after seven days in all samples taken1.
Quality, Comfort & Safety
The Careline+™ range of products are Unomedical’s (a ConvaTec Company) continence solution. Designed to provide patients with a range of high quality products that are discrete, comfortable & safe, the Careline+™ range of products aim to deliver value for patients, carers, healthcare professionals and NHS commissioners.
For information only, products are not available in Singapore.
WSACS (World Society of Abdominal Compartment Syndrome) recommends that patients should be screened for IAH/ACS risk factors upon ICU admission and in the presence of new or progressive organ failure. WSACS has generated consensus definitions and shares knowledge on diagnosis, management and treatment of IAH and ACS.

Polyvinyl chloride (PVC) plastic is used in medical devices for critical applications such as enternal feeding, wound drainage and airway ventilation. However, it is hard and brittle at room temperature. To make it flexible, it must be softened by a chemical additive called a "plasticizer."
The most commonly used plasticizers today are phthalates.
DEHP (Di-(2-ethylhexyl) phthalate), also known as dioctyl phthalate of DOP, has been the most commonly used phthalate plasticizer in the medical device industry.2
1. Ashman P, SMTL Closed System Study Test Report, 2009, Data on file, ConvaTec Inc.
2. Scientific Committee on Emerging and Newly-Identified Health Risks (SCENIHR) Opinion on the safety of medical devices
containing DEHP plasticised PVC or other plasticisers on neonates and other groups possibly at risk - 6 February 2008.